Crystallization Chemistry Lab


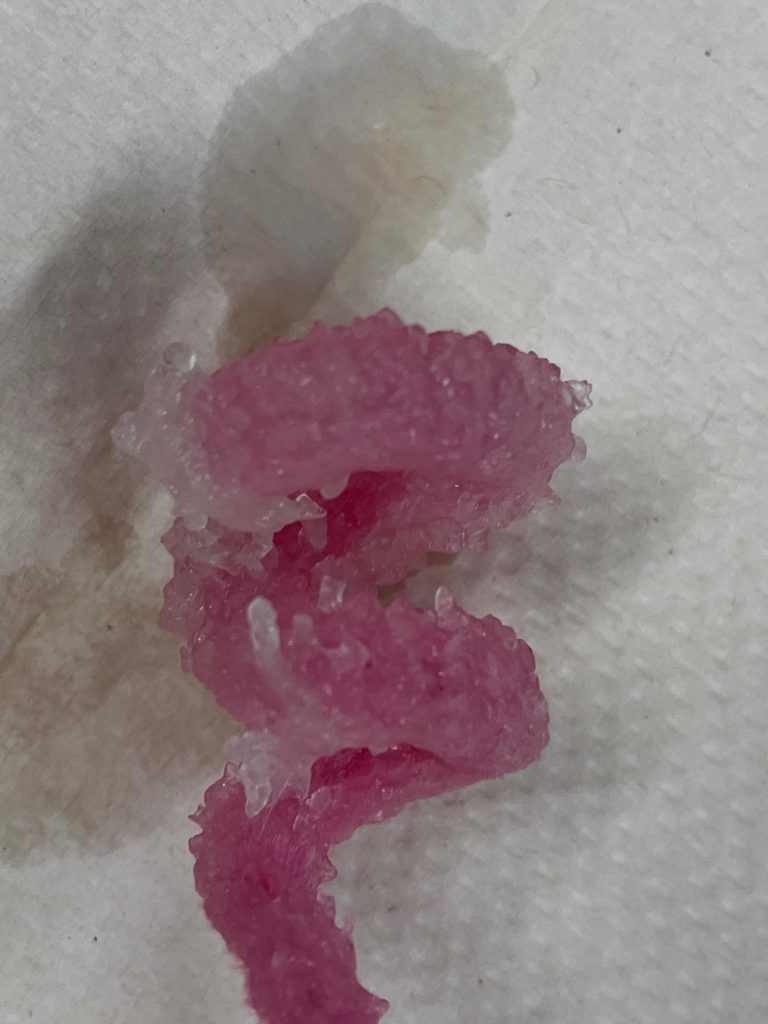





Last week in class we learned about crystals and the process of crystallization. A crystal is a solid substance with a symmetrical pattern of faces and angles with a repetitive arrangement of atoms in a substance. Snowflakes are one type of crystal. They are formed when air and water cool and molecules move closer together. Crystallization is a natural process when the materials solidify from liquids, or they precipitate out of liquid or gas. Crystals can be made of 1 species of atom, different species of ions, or even from molecules like proteins.
During the lab we learned how a solute(borax) and solvent(water) interact to form crystals. We also learned that different conditions affect how crystals grow. We tested making crystals from different materials like epsom salt, paperclips and string in a solution. During the experiments we discovered that materials like epsom salt and paperclips do not provide the proper conditions to grow a crystal, but borax, pipe cleaners and string do. We also noticed that the crystals that grew on the string were larger than the crystals that grew on the pipe cleaner.
Following the lab, we had an opportunity to record our observations and use magnifying glasses to determine the shape and size of our crystals. We learned that crystals grow together and the surfaces are either be flat or curved. We also learned how to define unsaturated, saturated, and supersaturated solutions and were able to point out in the lab when our solution went through each phase.
Blog Post Written By: A.W