Chapter 4: Atomic Theory
Lab Activity #1:
Mini Lab on Classification of elements
 Loading...
Loading...
Lab Activity #2:

Lab Activity #3:
Lab 4-3A “Conservation of Mass” from BC Science 10 Textbook Page 203
Sc. 10 Lab. 4-3A Conservation of Mass data
Chapter 5: Classifying Compounds
Lab Activity #1:
BC Sc. 10 Lab 5-1A “Acidic, Basic or Neutral?”; Page 221.
Total = X/Formative; (Practice using indicators to test pH);
Learning Goal: To be able to explain how you know a solution is acidic, basic or neutral.
Lab Activity #2:
Student Lab Sheet: “Properties of Acids & Bases”
 Loading...
.
Loading...
.
 Loading...
Loading...
Total = X/20
Claim, evidence Reasoning Conclusion. Be able to explain “how you know the pH of an unknown solution.
Student Copy lab 5-1b “Properties of Acids and Bases”
Student copy of Lab 5-1B Conclusion Writing Sheet
Lab Activity #3:
BC Sc. 10 Lab 5-2A”Three Salts”; Page 235.
Total = x/10
Results & Conclusion. Be able to explain how salts are produced (Reactants to products).
Student Copy lab 5-3B “Three Salts”
Video #1: Acid + Base react to produce a salt and water and heat
Neutralization Reactions:
Video #2: Acid + Metal react to produce a salt and hydrogen gas
Video #3: Acid + metal carbonate react to produce a salt, water and carbon dioxide
Lab Activity #4:
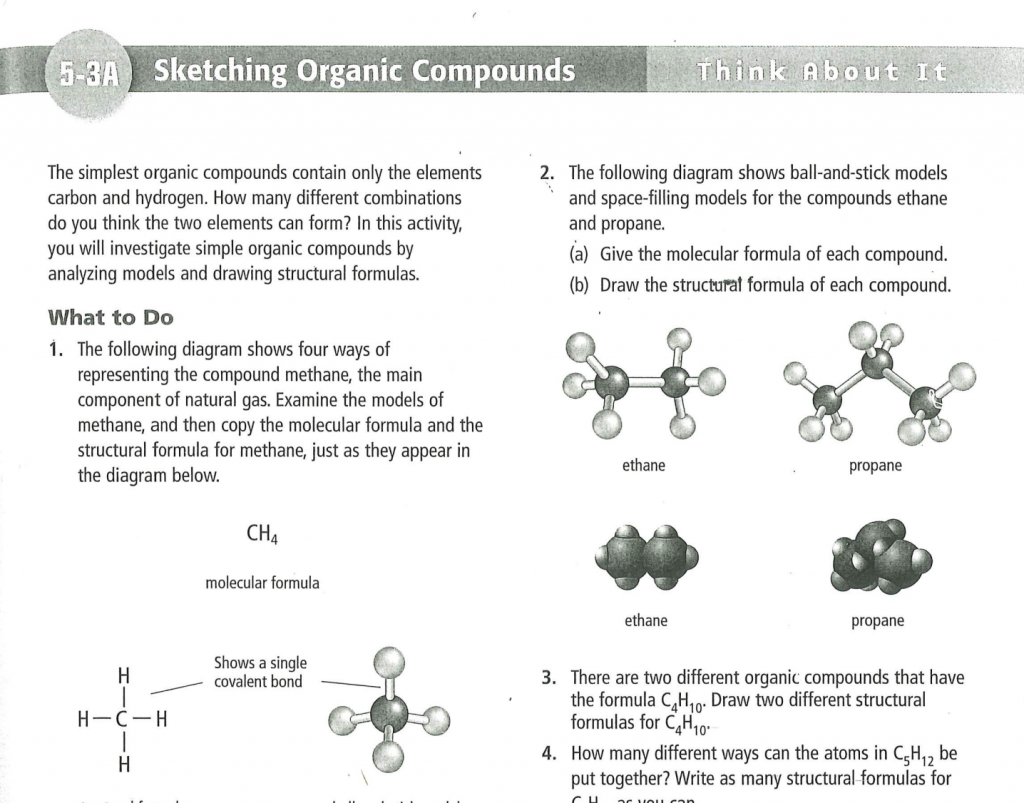
BC Sc. 10 Lab 5-3A “Sketching Organic Compounds” Page 245.
Total = X/Formative
Answer the questions in “What did you find out? Page 245.
Lab Activity #5:
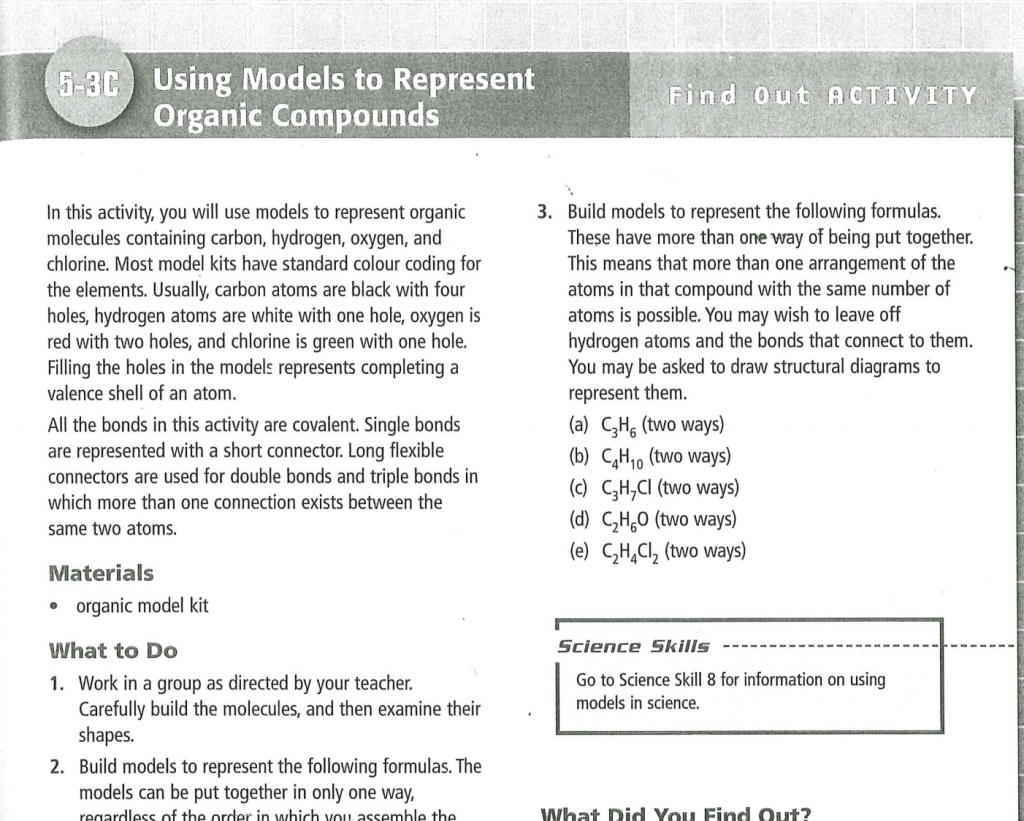
BC Sc. 10 Lab 5-3c “Using Models to Represent Organic Compounds” Page 249.
Total = X/Formative
Answer questions on Page 249 “What did you find out”.
Lab Activity #6:
Lab #1:
 Loading...
Loading...
Reaction rates
LAB 6A: Reaction Types & How to speed up reactions
Making Bath Bombs_Practice Predicting the products:
 Loading...
Loading...
Chapter 7: Atomic Theory
Virtual Field Trip – Manhattan Project